

The sequence of events that result in the contraction of an individual muscle fiber begins with a signal — the neurotransmitter, acetylcholine (ACh) — from the motor neuron innervating that fiber. When an action potential traveling down the motor neuron arrives at the neuromuscular junction ACh is released from the axon terminal. These ACh molecules bind to receptors on the motor end plate (the specialized sarcolemma at the neuromuscular junction). This binding leads to the opening of sodium ion channels on the motor end plate and causes the sarcolemma to depolarize as positively charged sodium ions (Na + ) enter, triggering an action potential that spreads to the rest of the membrane, including the T-tubules. This triggers the release of calcium ions (Ca ++ ) from storage in the sarcoplasmic reticulum (SR). The Ca ++ then initiates contraction by binding to a thin filament regulatory protein (troponin) causing a molecular interaction that moves another thin filament regulatory protein (tropomyosin) off the myosin binding sites on actin. As soon as the myosin binding sites are exposed, myosin heads bind to actin and move through a “cross-bridge cycle”, that leads to muscle contraction (Figure \(\PageIndex\)). As long as Ca ++ ions remain in the sarcoplasm to bind to troponin, which keeps the actin-binding sites “unshielded,” and as long as ATP is available to drive the cross-bridge cycling and the pulling of actin strands by myosin, the muscle fiber will continue to shorten to an anatomical limit.
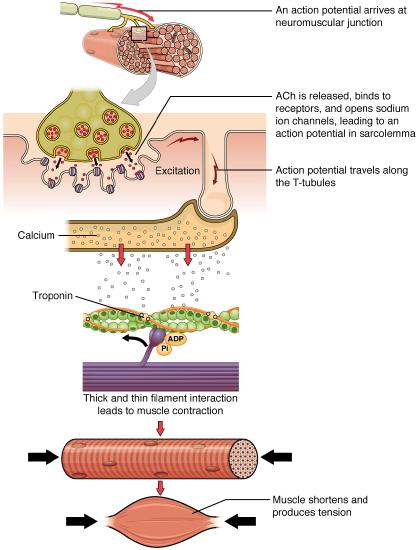
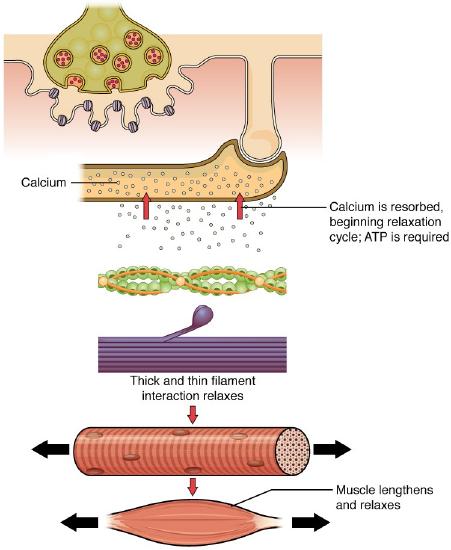
Muscle contraction usually stops when signaling from the motor neuron ends, which repolarizes the sarcolemma and T-tubules, and closes the voltage-gated calcium channels in the SR. Ca ++ ions are then pumped back into the SR, through the process of active transport, which requires ATP. The lack of Ca ++ ions causes the tropomyosin to reshield (or re-cover) the binding sites on the actin strands, allowing the actin (thin) and myosin (thick) interaction to relax, ending the cross-bridge cycle. This leads to the muscle relaxing and lengthening. A muscle also can stop contracting when it runs out of ATP and becomes fatigued (Figure \(\PageIndex\)).
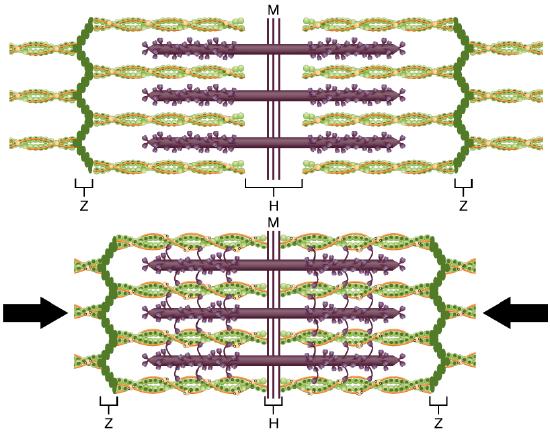
The molecular events of muscle fiber shortening occur within the fiber’s sarcomeres (see Figure \(\PageIndex\)). The contraction of a striated muscle fiber occurs as the sarcomeres, linearly arranged within myofibrils, shorten as myosin heads pull on the actin filaments.
The region where thick and thin filaments overlap has a dense appearance, as there is little space between the filaments. This zone where thin and thick filaments overlap is very important to muscle contraction, as it is the site where filament movement starts. Thin filaments, anchored at their ends by the Z-discs, do not extend completely into the central region that only contains thick filaments (H-zone), anchored at their bases at the M-line. A myofibril is composed of many sarcomeres running along its length; thus, myofibrils and muscle cells contract as the sarcomeres contract.
When signaled by a motor neuron, a skeletal muscle fiber contracts as the thin filaments are pulled and then slide past the thick filaments within the fiber’s sarcomeres. This process is known as the sliding filament model of muscle contraction (Figure \(\PageIndex\)). The sliding can only occur when myosin-binding sites on the actin filaments are exposed by a series of steps that begins with Ca ++ entry into the sarcoplasm.
Tropomyosin is a protein that winds around the chains of the actin filament and covers the myosin-binding sites to prevent actin from binding to myosin. Tropomyosin binds to troponin, which anchors the tropomyosin in place, to form a troponin-tropomyosin complex. In a relaxed muscle, the troponin-tropomyosin complex prevents the myosin heads from binding to the active sites on the actin microfilaments. Troponin also has a binding site for Ca ++ ions.
These two regulatory proteins work together to respond to calcium and thus “regulate” sarcomere contraction. To initiate muscle contraction, the position of tropomyosin is shifted to expose the myosin-binding site on an actin filament to allow cross-bridge formation between the actin and myosin microfilaments. The first step in the process of contraction is for Ca ++ to bind to troponin causing an interaction that slides tropomyosin away from the binding sites on actin filaments. This allows the myosin heads to bind to these exposed binding sites and form cross-bridges. The thin filaments are then pulled by the myosin heads to slide past the thick filaments toward the center of the sarcomere. But each head can only pull a very short distance before it has reached its limit and must be “re-cocked” before it can pull again, a step that requires ATP.
For thin filaments to continue to slide past thick filaments during muscle contraction, myosin heads must pull the actin at the binding sites, detach, re-cock, attach to more binding sites, pull, detach, re-cock, etc. This repeated movement is known as the cross-bridge cycle. This motion of the myosin heads is similar to the oars when an individual rows a boat: The paddle of the oars (the myosin heads) pull, are lifted from the water (detach), repositioned (re-cocked) and then immersed again to pull (Figure \(\PageIndex\)). Each cycle requires energy, and the action of the myosin heads in the sarcomeres repetitively pulling on the thin filaments also requires energy, which is provided by ATP.
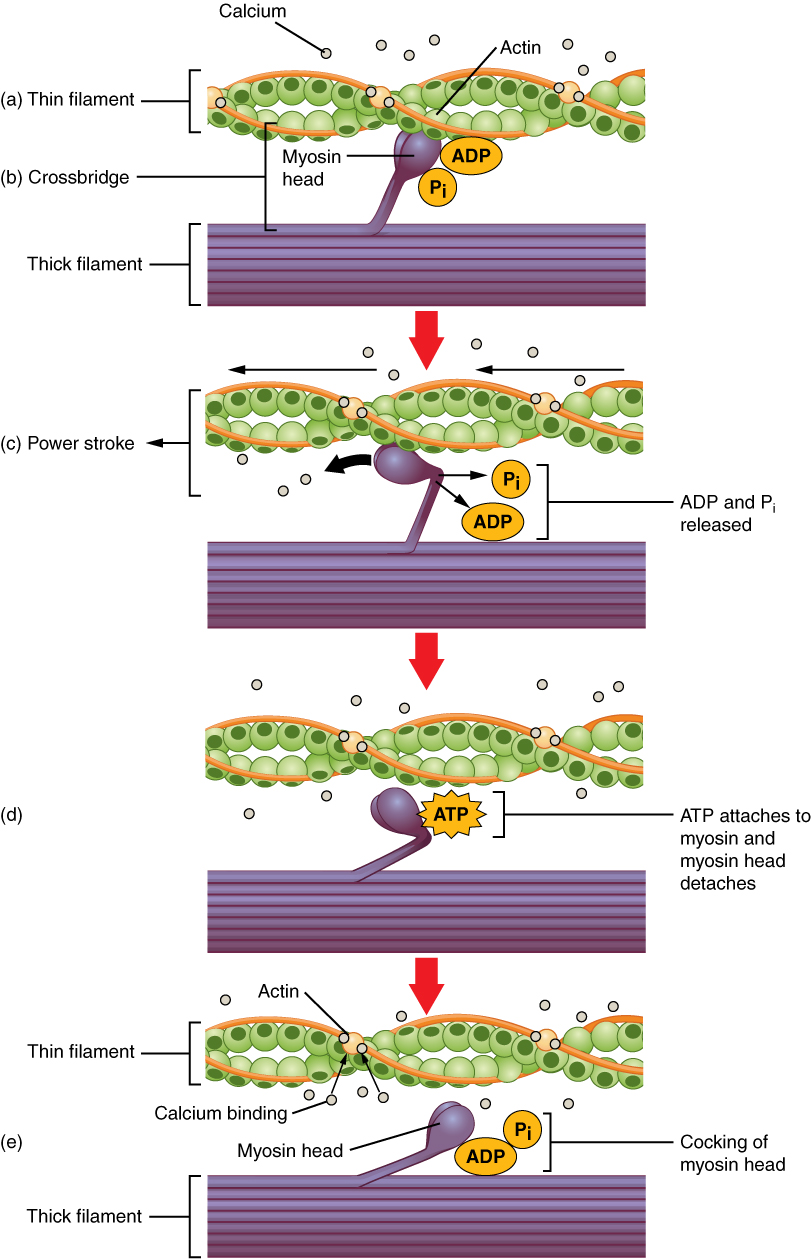
Cross-bridge formation occurs when the myosin head attaches to actin while adenosine diphosphate (ADP) and inorganic phosphate (Pi) are still bound to myosin (Figure \(\PageIndex\).a,b.). Pi is then released, causing myosin to form a stronger attachment to the actin, after which the myosin head moves toward the M-line, pulling the actin along with it. As actin is pulled, the filaments move approximately 10 nm toward the M-line. This movement is called the power stroke, as movement of the thin filament occurs at this step (Figure \(\PageIndex\).c.). In the absence of ATP, the myosin head will not detach from actin.
In addition to the actin binding sites on myosin heads, there is also an ATP binding site. When ATP binds in this location, it causes the myosin head to detach from the actin (Figure \(\PageIndex\).d). After this occurs, ATP is converted to ADP and Pi by the intrinsic ATPase activity of myosin. The energy released during ATP hydrolysis changes the angle of the myosin head into a cocked position (Figure \(\PageIndex\).e). The myosin head is now in position for further movement.
When the myosin head is cocked, myosin is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke, and at the end of the power stroke, the myosin head is in a low-energy position. After the power stroke, ADP is released; however, the formed cross-bridge is still in place, and actin and myosin are bound together. As long as ATP is available, it readily attaches to myosin, the cross-bridge cycle can recur, and muscle contraction can continue.
Note that each thick filament of roughly 300 myosin molecules has multiple myosin heads, and many cross-bridges form and break continuously during muscle contraction. Multiply this by all of the sarcomeres in one myofibril, all the myofibrils in one muscle fiber, and all of the muscle fibers in one skeletal muscle, and you can understand why so much energy (ATP) is needed to keep skeletal muscles working. In fact, it is the loss of ATP that results in the rigor mortis observed soon after someone dies. With no further ATP production possible, there is no ATP available for myosin heads to detach from the actin-binding sites, so the cross-bridges stay in place, causing the rigidity in the skeletal muscles.
Relaxing skeletal muscle fibers, and ultimately, the skeletal muscle, begins with the motor neuron, which stops releasing its chemical signal, ACh, into the synapse at the NMJ. The muscle fiber will repolarize, which closes the gates in the SR where Ca ++ was being released. ATP-driven pumps will move Ca ++ out of the sarcoplasm back into the SR. This results in the “reshielding” of the actin-binding sites on the thin filaments. Without the ability to form cross-bridges between the thin and thick filaments, the muscle fiber loses its tension and relaxes.
The number and type of skeletal muscle fibers in a given muscle is genetically determined and does not change. Muscle strength is directly related to the amount of myofibrils and sarcomeres within each fiber. Factors, such as hormones and stress (and artificial anabolic steroids), acting on the muscle can increase the production of sarcomeres and myofibrils within the muscle fibers, a change called hypertrophy, which results in an increased mass and bulk of a skeletal muscle. Likewise, decreased use of a skeletal muscle results in atrophy, where the number of sarcomeres and myofibrils decrease (but not the number of muscle fibers). It is common for a limb in a cast to result in dramatically atrophied muscles and certain diseases, such as polio, present with muscular atrophy as a comorbidity.
DISORDERS OF THE.
Muscular System
Duchenne muscular dystrophy (DMD) is a progressive weakening of the skeletal muscles. It is one of several diseases collectively referred to as “muscular dystrophy.” DMD is caused by a lack of the protein dystrophin, which helps the thin filaments of myofibrils bind to the sarcolemma. Without sufficient dystrophin, muscle contractions cause the sarcolemma to tear, causing an influx of Ca++, leading to cellular damage and muscle fiber degradation. Over time, as muscle damage accumulates, muscle mass is lost, and greater functional impairments develop.
DMD is an inherited disorder caused by an abnormal X chromosome. It primarily affects males, and it is usually diagnosed in early childhood. DMD usually first appears as difficulty with balance and motion, and then progresses to an inability to walk. It continues progressing upward in the body from the lower extremities to the upper body, where it affects the muscles responsible for breathing and circulation. It ultimately causes death due to respiratory failure, and those afflicted do not usually live past their 20s.
Because DMD is caused by a mutation in the gene that codes for dystrophin, it was thought that introducing healthy myoblasts into patients might be an effective treatment. Myoblasts are the embryonic cells responsible for muscle development, and ideally, they would carry healthy genes that could produce the dystrophin needed for normal muscle contraction. This approach has been largely unsuccessful in humans. A more recent approach has involved attempting to boost the muscle’s production of utrophin, a protein similar to dystrophin that may be able to assume the role of dystrophin and prevent cellular damage from occurring.
A muscle cell is filled with thousands of myofibrils, chains of sarcomeres attached from Z-disc to Z-disc. Sarcomeres are the smallest contractile portion of a muscle. Myofibrils are composed of thick and thin filaments. Thick filaments are composed of the protein myosin; thin filaments are composed of the protein actin. Troponin and tropomyosin are regulatory proteins affiliated with the thin filament.
Muscle contraction is described by the sliding filament model of contraction. ACh is the neurotransmitter that binds at the neuromuscular junction (NMJ) to trigger depolarization, and an action potential travels along the sarcolemma to trigger calcium release from the SR. The myosin binding sites on actin sites are exposed after calcium enters the sarcoplasm and activates the troponin-tropomyosin complex to shift. The latching of myosin heads docking onto actin-binding sites begins the “cross bridge cycle” a process that continues as long as calcium and ATP are present. During the “power stroke” myosin pulls thin filaments towards the M-line and as thin filaments slide over thick filaments, the Z-discs throughout a myofibril draw closer together to shorten the entire muscle fiber. Ultimately, the sarcomeres, myofibrils, and muscle fibers shorten to produce skeletal muscle contraction.
Q. In relaxed muscle, the myosin-binding site on actin is blocked by ________.
Answer
Q. According to the sliding filament model, binding sites on actin open when ________.
A. creatine phosphate levels rise
B. ATP levels rise
C. acetylcholine levels rise
D. calcium ion levels rise
Answer
Q. The cell membrane of a muscle fiber is called ________.
Answer
Q. Muscle relaxation occurs when ________.
A. calcium ions are actively transported out of the sarcoplasmic reticulum
B. calcium ions diffuse out of the sarcoplasmic reticulum
C. calcium ions are actively transported into the sarcoplasmic reticulum
D. calcium ions diffuse into the sarcoplasmic reticulum
Answer
Q. During muscle contraction, the cross-bridge detaches when ________.
A. an ADP molecule binds to the myosin head
B. an ATP molecule binds to the myosin head
C. calcium ions bind to troponin
D. calcium ions bind to actin
Answer
Q. Thin and thick filaments are organized into functional units called ________.
Answer
Q. How would muscle contractions be affected if skeletal muscle fibers did not have T-tubules?
Answer
A. Without T-tubules, action potential conduction into the interior of the cell would happen much more slowly, causing delays between neural stimulation and muscle contraction, resulting in slower, weaker contractions.
Q. What causes the striated appearance of skeletal muscle tissue?
Answer
A. The microscopic arrangement of myofilaments within sarcomeres results in dark A bands and light I bands. These bands repeat along myofibrils, and the alignment of myofibrils in the cell cause the entire cell to appear striated.
Q. How would muscle contractions be affected if ATP was completely depleted in a muscle fiber?
Answer
A. Without ATP, the myosin heads cannot detach from the actin-binding sites. All of the “stuck” cross-bridges result in muscle stiffness. In a live person, this can cause a condition like “writer’s cramps.” In a recently dead person, it results in rigor mortis.
aerobic respiration production of ATP in the presence of oxygen ATPase enzyme that hydrolyzes ATP to ADP creatine phosphate phosphagen used to store energy from ATP and transfer it to muscle glycolysis anaerobic breakdown of glucose to ATP lactic acid product of anaerobic glycolysis oxygen debt amount of oxygen needed to compensate for ATP produced without oxygen during muscle contraction power stroke action of myosin pulling actin inward (toward the M line) pyruvic acid product of glycolysis that can be used in aerobic respiration or converted to lactic acid troponin regulatory protein that binds to actin, tropomyosin, and calcium tropomyosin regulatory protein that covers myosin-binding sites to prevent actin from binding to myosin